Preliminary performance screening of microbial consortia on fusarium basal rot control and shallot growth
Main Article Content
Abstract
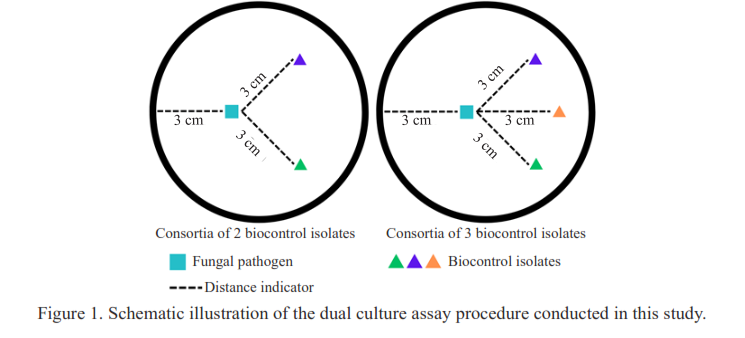
Developing an effective biocontrol consortium requires comprehensive assessment to ensure that the selected microbial combinations can provide both strong disease suppression and plant growth-promoting effects. This study aimed to evaluate the performance of four biocontrol consortia composed of indigenous microbes from Bantul Regency, Indonesia, in suppressing Fusarium basal rot (FBR) and promoting the growth of shallot (Allium cepa var. aggregatum) cv. Bauji. Three indigenous isolates were used: Trichoderma asperellum strain PBt1, Bacillus cereus strain PBt2, and B. cereus strain PBt3. Four consortia were formulated by combining two or three of these isolates, designated as Consortia A, B, C, and D. The biocontrol activity against Fusarium solani DRB-1 was evaluated for both single isolates and consortia. A greenhouse experiment was conducted using a Completely Randomized Design with two inoculation timings (before planting and early vegetative stage) and five replicates. The performance of each consortium was assessed based on FBR severity and shallot growth parameters. Results showed that Consortia B (T. asperellum PBt1 + B. cereus PBt3) applied before planting achieved the highest FBR reduction (34.8%) at 42 days after planting (DAP). Moreover, this consortium significantly enhanced shallot yield, as reflected by increased bulb weight and number. These findings suggest that Consortia B has strong potential to improve both FBR management efficacy and shallot productivity.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Aisyah SN, Harnas H, Sulastri S, Retmi R, Fuaddi H, Fatchiyah F, Bactiar A, & Jamsari J. 2016. Enhancement of a novel isolate of Serratia plymuthica as potential candidate for an antianthracnose. Pak. J. Biol. Sci. 9(6): 250–258. https://doi.org/10.3923/pjbs.2016.250.258
Aisyah SN, Khoiruddin M, Hidayat T, & Astuti A. 2022. Comparison on Fusarium basal rot occurrence among shallot cultivations in Bantul regency. IOP Conf. Ser.: Earth Environ. Sci. 985: 012052. https://doi.org/10.1088/1755-1315/985/1/012052
Anbalagan SA, Appusamy S, Kumaresan PV, Chellappan G, Narayanan S, Rangasamy A, Parveen K, Bukhari NA, & Sayyed R. 2024. Deciphering the biocontrol potential of Trichoderma asperellum (Tv1) against Fusarium‐nematode wilt complex in tomato. J. Basic Microbiol. 65(1): e2400595. https://doi.org/10.1002/jobm.202400595
Ankati S, Srinivas V, Pratyusha S, & Gopalakrishnan S. 2021. Streptomyces consortia-mediated plant defense against Fusarium wilt and plant growth-promotion in chickpea. Microb. Pathog. 157: 104961. https://doi.org/10.1016/j.micpath.2021.104961
Asrul A. 2023. Potential of local Bacillus spp. isolates as wilt disease biocontrol agents for Fusarium oxysporum f.sp. cepae on Allium x wakegi. Biodiversitas. 24(9): 4989–4997. https://doi.org/10.13057/biodiv/d240942
Báez-Astorga PA, Cázares-Álvarez JE, Cruz-Mendívil A, Quiroz-Figueroa FR, Sánchez-Valle VI, & Maldonado-Mendoza IE. 2022. Molecular and biochemical characterisation of antagonistic mechanisms of the biocontrol agent Bacillus cereus B25 inhibiting the growth of the phytopathogen Fusarium verticillioides P03 during their direct interaction in vitro. Biocontrol Sci. Technol. 32(9): 1074–1094. https://doi.org/10.1080/09583157.2022.2085662
Bhatia SK, Yoon JJ, Kim HJ, Hong JW, Hong YG, Song HS, Moon YM, Jeon JM, Kim YG, & Yang YH. 2018. Engineering of artificial microbial consortia of Ralstonia eutropha and Bacillus subtilis for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production from sugarcane sugar without precursor feeding. Bioresour. Technol. 257: 92–101. https://doi.org/10.1016/j.biortech.2018.02.056
Bhatt P, Bhatt K, Sharma A, Zhang W, Mishra S, & Chen S. 2021. Biotechnological basis of microbial consortia for the removal of pesticides from the environment. Crit. Rev. Biotechnol. 41(3): 317–338. https://doi.org/10.1080/07388551.2020.1853032
Bunbury-Blanchette AL & Walker AK. 2019. Trichoderma species show biocontrol potential in dual culture and greenhouse bioassays against Fusarium basal rot of onion. Biol. Control. 130: 127–135. https://doi.org/10.1016/j.biocontrol.2018.11.007
Cahyaningrum H, Suryanti, & Widiastuti A. 2020. Response and resistance mechanism of shallot var. Topo, a north molluca’s local variety against basal rot disease. Paper presented at the 5th International Conference on Food, Agriculture and Natural Resources (FANRes 2019). pp. 71–75. https://doi.org/10.2991/aer.k.200325.015
Cai X, Zhao H, Liang C, Li M, & Liu R. 2021. Effects and mechanisms of symbiotic microbial combination agents to control tomato Fusarium crown and root rot disease. Front. Microbiol.12: 629793. https://doi.org/10.3389/fmicb.2021.629793
Degani O, Dimant E, Gordani A, Graph S, & Margalit E. 2022. Prevention and control of Fusarium spp., the causal agents of onion (Allium cepa) basal rot. Horticulturae. 8(11): 1071. https://doi.org/10.3390/horticulturae8111071
Dewi FS, Dewi RR, Abadi AL, Setiawan A, Aini LQ, & Syib’li MA. 2025. Biocontrol of Fusarium oxysporum f.sp. cepae on Indonesian local garlic plants (Lumbu Hijau) using a consortium of Bacillus amyloliquefaciens B1 and arbuscular mycorrhizal fungi. Mycobiology. 53(1): 1–9. https://doi.org/10.1080/12298093.2024.2433826
Diabankana RGC, Frolov M, Islamov B, Shulga E, Filimonova MN, Afordoanyi DM, & Validov S. 2024. Identification and aggressiveness of Fusarium species associated with onion bulb (Allium cepa L.) during storage. J. Fungi. 10(2): 161. https://doi.org/10.3390/jof10020161
El-Komy MH, Al-Qahtani RM, Ibrahim YE, Almasrahi AA, & Al-Saleh MA. 2022. Soil application of Trichoderma asperellum strains significantly improves Fusarium root and stem rot disease management and promotes growth in cucumbers in semi-arid regions. Eur. J. Plant Pathol. 162(3): 637–653. https://doi.org/10.1007/s10658-021-02427-0
Fierer N, Wood SA, & de Mesquita CPB. 2021. How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 153: 108111. https://doi.org/10.1016/j.soilbio.2020.108111
Galván GA, Koning-Boucoiran CF, Koopman WJ, Burger-Meijer K, González PH, Waalwijk C, Kik C, & Scholten OE. 2008. Genetic variation among Fusarium isolates from onion, and resistance to Fusarium basal rot in related Allium species. Eur. J. Plant Pathol. 121(4): 499–512. https://doi.org/10.1007/s10658-008-9270-9
Ganuza M, Pastor N, Erazo J, Andrés J, Reynoso MM, Rovera M, & Torres AM. 2018. Efficacy of the biocontrol agent Trichoderma harzianum ITEM 3636 against peanut smut, an emergent disease caused by Thecaphora frezii. Eur. J. Plant Pathol. 151: 257–262. https://doi.org/10.1007/s10658-017-1360-0
Giordano DF, Pastor NA, Rouws LFM, de Freitas KM, Erazo JG, Canto AD, Coelho IdS, Oddino CM. & Torres AM. 2023. Trichoderma harzianum ITEM 3636 colonizes peanut roots as an endophyte and protects the plants against late leaf spot. Symbiosis. 89(3): 337–352. https://doi.org/10.1007/s13199-023-00913-z
Grünwald NJ, Hu S, & van Bruggen A. 2000. Short-term cover crop decomposition in organic and conventional soils: Characterization of soil C, N, microbial and plant pathogen dynamics. Eur. J. Plant Pathol. 106(1): 37–50. https://doi.org/10.1023/A:1008720731062
Guzmán-Guzmán P, Kumar A, de Los Santos-Villalobos S, Parra-Cota FI, Orozco-Mosqueda MdC, Fadiji AE, Hayder S, Babalola OO, & Santoyo G. 2023. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants. 12(3): 432. https://doi.org/10.3390/plants12030432
He A, Sun J, Wang X, Zou L, Fu B, & Chen J. 2019. Reprogrammed endophytic microbial community in maize stalk induced by Trichoderma asperellum biocontrol agent against Fusarium diseases and mycotoxin accumulation. Fungal Biol. 123(6): 448–455. https://doi.org/10.1016/j.funbio.2019.03.003
Herlina L, Istiaji B, & Wiyono S. 2021. The causal agent of Fusarium disease infested shallots in Java Islands of Indonesia. E3S Web Conf. 232: 03003. https://doi.org/10.1051/e3sconf/202123203003
Islam MR, Jeong YT, Lee YS, & Song CH. 2012. Isolation and identification of antifungal compounds from Bacillus subtilis C9 inhibiting the growth of plant pathogenic fungi. Mycobiol. 40(1): 59–65. https://doi.org/10.5941/MYCO.2012.40.1.059
Izquierdo-García LF, Cotes AM, & Moreno-Velandia C. 2021. Screening for effective microbial consortia against fusarium wilt of cape gooseberry (Physalis peruviana). BioControl. 66(5): 713–725. https://doi.org/10.1007/s10526-021-10095-6
Izquierdo-García LF, González-Almario A, Cotes AM, & Moreno-Velandia C. 2020. Trichoderma virens Gl006 and Bacillus velezensis Bs006: A compatible interaction controlling fusarium wilt of cape gooseberry. Sci. Rep. 10(1): 6857. https://doi.org/10.1038/s41598-020-63689-y
Jamil A, Musheer N, & Ashraf S. 2021. Antagonistic potential of Trichoderma harzianum and Azadirachta indica against Fusarium oxysporum f.sp. capsici for the management of chilli wilt. J. Plant Dis. Prot. 128(1): 161–172. https://doi.org/10.1007/s41348-020-00383-1
Jangir M, Sharma S, & Sharma S. 2019. Target and non-target effects of dual inoculation of biocontrol agents against fusarium wilt in Solanum lycopersicum. Biol. Control. 138: 104069. https://doi.org/10.1016/j.biocontrol.2019.104069
Kaleh AM, Singh P, Mazumdar P, Wong GR, Chua KO, & Harikrishna JA. 2023. A halotolerant plant growth promoting consortium of Bacillus sp. RB3 and Pseudomonas sp. EB3 primes banana, Musa acuminata cv. Berangan, against salinity and Foc-TR4 stresses. Curr. Plant Biol. 35: 100294. https://doi.org/10.1016/j.cpb.2023.100294
Kalman B, Abraham D, Graph S, Perl-Treves R, Harel YM, & Degani O. 2020. Isolation and identification of Fusarium spp., the causal agents of onion (Allium cepa) basal rot in Northeastern Israel. Biology. 9(4): 69. https://doi.org/10.3390/biology9040069
Kara M, Soylu S, Gümüş Y, Soylu EM, Uysal A, & Kurt Ş. 2023. Determination of in vitro biocontrol potentials of antagonist bacterial isolates against onion basal and root rot disease agent Fusarium proliferatum. IJIAAR. 7(4): 487–497. https://doi.org/10.29329/ijiaar.2023.630.10
Karim H, Azis AA, & Jumadi O. 2022. Antagonistic activity and characterization of indigenous soil isolates of bacteria and fungi against onion wilt incited by Fusarium sp. Arch. Microbiol. 204(1): 68. https://doi.org/10.1007/s00203-021-02663-2
Karuppiah V, He A, Lu Z, Wang X, Li Y, & Chen J. 2022. Trichoderma asperellum GDFS1009‐mediated maize resistance against Fusarium graminearum stalk rot and mycotoxin degradation. Biol. Control. 174: 105026. https://doi.org/10.1016/j.biocontrol.2022.105026
Kaur S, Samota MK, Choudhary M, Choudhary M, Pandey AK, Sharma A, & Thakur J. 2022. How do plants defend themselves against pathogens-biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants. 28(2): 485–504. https://doi.org/10.1007/s12298-022-01146-y
Krestini EH, Rusmawati U, & Susilawati A. 2020. Effectiveness of microbial consortium on growth, yield, and intensity of withered disease (Fusarium oxysporum Schelecht) on garlic plants. BIO Web Conf. 20: 03009. https://doi.org/10.1051/bioconf/20202003009
Kumar S, Shukla V, Dubey MK, & Upadhyay RS. 2021. Activation of defense response in common bean against stem rot disease triggered by Trichoderma erinaceum and Trichoderma viride. J. Basic Microbiol. 61(10): 910–922. https://doi.org/10.1002/jobm.202000749
Le D, Ameye M, De Boevre M, De Saeger S, Audenaert K, & Haesaert G. 2021. Population, virulence, and mycotoxin profile of Fusarium spp. associated with basal rot of Allium spp. in Vietnam. Plant Dis. 105(7): 1942–1950. https://doi.org/10.1094/PDIS-08-20-1850-RE
Lecomte C, Alabouvette C, Edel-Hermann V, Robert F, & Steinberg C. 2016. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control. 101: 17–30. https://doi.org/10.1016/j.biocontrol.2016.06.004
Lestiyani A, Wibowo A, Subandiyah S, Gambley C, Ito S, & Harper S. 2014. Identification of Fusarium spp., the causal agent of twisted disease of shallot. Paper presented at the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014). 1128. pp. 155–160. https://doi.org/10.17660/ActaHortic.2016.1128.22
Li J, Philp J, Li J, Wei Y, Li H, Yang K, Ryder M, Toh R, Zhou Y, Denton MD, Hu J, & Wang Y. 2020. Trichoderma harzianum inoculation reduces the incidence of clubroot disease in chinese cabbage by regulating the rhizosphere microbial community. Microorganisms. 8(9): 1325. https://doi.org/10.3390/microorganisms8091325
Li XG, Ding CF, Hua K, Zhang TL, Zhang YN, Zhao L, Yang YR, Liu JG, & Wang XX. 2014. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 78: 149–159. https://doi.org/10.1016/j.soilbio.2014.07.019
Liu H, Li T, Li Y, Wang X, & Chen J. 2022. Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the biocontrol of wheat head blight. J. Fungi. 8(12): 1250. https://doi.org/10.3390/jof8121250
Lucas P. 2006. Diseases caused by soil-borne pathogens. In: Cooke B, Jones D, & Kaye B (Eds.). The Epidemiology of Plant Diseases. pp. 373–386. Dordrecht: Springer. Netherlands. https://doi.org/10.1007/1-4020-4581-6_14
Madriz-Ordeñana K, Pazarlar S, Jørgensen HJL, Nielsen TK, Zhang Y, Nielsen KL, Hansen LH, & Thordal-Christensen H. 2022. The Bacillus cereus strain EC9 primes the plant immune system for superior biocontrol of Fusarium oxysporum. Plants. 11(5): 687. https://doi.org/10.3390/plants11050687
Marianah L, Nawangsih AA, Munif A, Giyanto G, & Tondok ET. 2024. Variation in symptoms and morphology of Fusarium spp. on shallot associated with basal plate rot disease in Brebes District, Central Java Province, Indonesia. Biodiversitas. 25(5): 2198–2208. https://doi.org/10.13057/biodiv/d250538
Meng T, Wang Q, Abbasi P, & Ma Y. 2019. Deciphering differences in the chemical and microbial characteristics of healthy and Fusarium wilt-infected watermelon rhizosphere soils. Appl. Microbiol. Biotechnol. 103(3): 1497–1509. https://doi.org/10.1007/s00253-018-9564-6
Minchev Z, Kostenko O, Soler R, & Pozo MJ. 2021. Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Front. Plant Sci. 12: 756368. https://doi.org/10.3389/fpls.2021.756368
Modrzewska M, Błaszczyk L, Stępień Ł, Urbaniak M, Waśkiewicz A, Yoshinari T, & Bryła M. 2022. Trichoderma versus Fusarium—Inhibition of pathogen growth and mycotoxin biosynthesis. Molecules. 27(23): 8146. https://doi.org/10.3390/molecules27238146
Nofal A, El-Rahman MA, Abdelghany T, & Abd El-Mongy M. 2021. Mycoparasitic nature of Egyptian Trichoderma isolates and their impact on suppression Fusarium wilt of tomato. Egypt. J. Biol. Pest Control. 31(1): 103. https://doi.org/10.1186/s41938-021-00450-1
Nunes PSO, Junior GVL, Mascarin GM, Guimarães RA, Medeiros FHV, Arthurs S, & Bettiol W. 2024. Microbial consortia of biological products: Do they have a future?. Biol. Control. 188: 105439. https://doi.org/10.1016/j.biocontrol.2024.105439
Palmieri D, Vitullo D, De Curtis F, & Lima G. 2017. A microbial consortium in the rhizosphere as a new biocontrol approach against fusarium decline of chickpea. Plant Soil. 412(1): 425–439. https://doi.org/10.1007/s11104-016-3080-1
Panchalingam H, Ashfield-Crook N, Naik V, Frenken R, Foster K, Tomlin R, Shapcott A, & Kurtböke Dİ. 2022. Testing the biocontrol ability of a Trichoderma-Streptomycetes consortium against Pyrrhoderma noxium (Corner) L.W. Zhou and Y.C. Dai in soil. J. Fungi. 9(1): 67. https://doi.org/10.3390/jof9010067
Pazarlar S, Madriz-Ordeñana K, & Thordal-Christensen H. 2022. Bacillus cereus EC9 protects tomato against Fusarium wilt through JA/ET-activated immunity. Front. Plant Sci. 13: 1090947. https://doi.org/10.3389/fpls.2022.1090947
Prigigallo MI, Cabanás CGL, Mercado-Blanco J, & Bubici G. 2022. Designing a synthetic microbial community devoted to biological control: The case study of fusarium wilt of banana. Front. Microbiol. 13: 967885. https://doi.org/10.3389/fmicb.2022.967885
Ram RM, Debnath A, Negi S, & Singh HB. 2022. Use of microbial consortia for broad spectrum protection of plant pathogens: Regulatory hurdles, present status and future prospects. In: Rakshit A, Meena VS, Abhilash Pc, Sarma Bk, Singh Hb, Fraceto L, Parihar M, Singh AK. Biopesticides. Volume 2: Advances in Bio-Inoculants. pp. 319–335. Elsevier. United Kingdom. https://doi.org/10.1016/B978-0-12-823355-9.00017-1
Ramírez V, Martínez J, Bustillos‐Cristales MR, Catañeda‐Antonio D, Munive JA, & Baez A. 2022. Bacillus cereus MH778713 elicits tomato plant protection against Fusarium oxysporum. J. Appl. Microbiol. 132(1): 470–482. https://doi.org/10.1111/jam.15179
Rao Y, Zeng L, Jiang H, Mei L, & Wang Y. 2022. Trichoderma atroviride LZ42 releases volatile organic compounds promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings. BMC Microbiol. 22(1): 88. https://doi.org/10.1186/s12866-022-02511-3
Resti Z, Warnita, & Liswarni Y. 2021. Endophytic bacterial consortia as biocontrol of purple blotch and plant growth promoters of shallots. IOP Conf. Ser.: Earth Environ. Sci. 741: 012009. https://doi.org/10.1088/1755-1315/741/1/012009
Romera FJ, García MJ, Lucena C, Martínez-Medina A, Aparicio MA, Ramos J, Alcántara E, Angulo M, & Pérez-Vicente R. 2019. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front. Plant Sci. 10: 287. https://doi.org/10.3389/fpls.2019.00287
Rosenzweig N, Tiedje JM, Quensen III JF, Meng Q, & Hao JJ. 2012. Microbial communities associated with potato common scab-suppressive soil determined by pyrosequencing analyses. Plant Dis. 96(5): 718–725. https://doi.org/10.1094/PDIS-07-11-0571
Sari GNP, Pustika AB, Solichah C, Wicaksono D, Widyayanti S, Sudarmaji S, & Yolanda K. 2023. The effect of antagonistic microbial and seed bulb-size on fusarium wilt and yield of shallot. E3S Web Conf. 467: 01006. https://doi.org/10.1051/e3sconf/202346701006
Sarma BK, Yadav SK, Singh S, & Singh HB. 2015. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 87: 25–33. https://doi.org/10.1016/j.soilbio.2015.04.001
Saxena AK, Kumar M, Chakdar H, Anuroopa N, & Bagyaraj DJ. 2020. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 128(6): 1583–1594. https://doi.org/10.1111/jam.14506
Sharma S, Mandal S, & Cramer CS. 2024. Recent advances in understanding and controlling Fusarium diseases of Alliums. Horticulturae. 10(5): 527. https://doi.org/10.3390/horticulturae10050527
Shen Z, Ruan Y, Xue C, Zhong S, Li R, & Shen Q. 2015. Soils naturally suppressive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil. 393(1): 21–33. https://doi.org/10.1007/s11104-015-2474-9
Shen Z, Wang D, Ruan Y, Xue C, Zhang J, Li R, & Shen Q. 2014. Deep 16S rRNA pyrosequencing reveals a bacterial community associated with banana fusarium wilt disease suppression induced by bio-organic fertilizer application. PLoS One. 9(5): e98420. https://doi.org/10.1371/journal.pone.0098420
Shin JH, Lee HK, Lee SC, & Han YK. 2023. Biological control of Fusarium oxysporum, the causal agent of Fusarium basal rot in onion by Bacillus spp. Plant Pathol. J. 39(6): 600–613. https://doi.org/10.5423/PPJ.OA.08.2023.0118
Nurcahyanti SD & Sholeh MI. 2023. Perkembangan penyakit moler (Fusarium oxysporum f.sp. cepae) pada sentra produksi bawang merah di Kabupaten Probolinggo. Berkala Ilmiah Pertanian. 6(2): 56–62. https://doi.org/10.19184/bip.v6i2.35392
Stummer BE, Zhang X, Yang H, & Harvey PR. 2022. Co-inoculation of Trichoderma gamsii A5MH and Trichoderma harzianum TR906 in wheat suppresses in planta abundance of the crown rot pathogen Fusarium pseudograminearum and impacts the rhizosphere soil fungal microbiome. Biol. Control. 165: 104809. https://doi.org/10.1016/j.biocontrol.2021.104809
Supyani S, Poromarto SH, Supriyadi S, & Hadiwiyono H. 2021. Moler disease of shallot in the last three years at Brebes Central Java: The intensity and resulting yields losses is increasing. IOP conference series: earth and environmental science. 810: 012004. https://doi.org/10.1088/1755-1315/810/1/012004
Sutariati GAK, Khaeruni A, Madiki A, Mudi L, Aco A, Ramadhani SA, Adri RM, & Mantja K. 2021. Effectiveness of endo-rhizobacteria as growth promoters and biological control of moler disease in shallots (Allium ascalonicum L.). IOP Conf. Ser.: Earth Environ. Sci. 681: 012028. https://doi.org/10.1088/1755-1315/681/1/012028
Sylla J, Alsanius BW, Krüger E, Becker D, & Wohanka W. 2013. In vitro compatibility of microbial agents for simultaneous application to control strawberry powdery mildew (Podosphaera aphanis). Crop Prot. 51: 40–47. https://doi.org/10.1016/j.cropro.2013.04.011
Taylor A, Vágány V, Jackson AC, Harrison RJ, Rainoni A, & Clarkson JP. 2016. Identification of pathogenicity‐related genes in Fusarium oxysporum f. sp. cepae. Mol. Plant Pathol. 17(7): 1032–1047. https://doi.org/10.1111/mpp.12346
Tirado-Ramírez MA, López-Orona CA, Díaz-Valdés T, Velarde-Félix S, Martínez-Campos AR, Romero-Gómez SJ, & Retes-Manjarrez JE. 2019. First report of basal rot of onion caused by Fusarium brachygibbosum in Sinaloa, Mexico. Plant Dis. 103(3): 582. https://doi.org/10.1094/PDIS-04-18-0710-PDN
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, & Prigent-Combaret C. 2013. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 4: 356. https://doi.org/10.3389/fpls.2013.00356
Wibowo A, Santika IA, Syafitri LM, Widiastuti A, Subandiyah S, & Harper S. 2023. Incidence of twisted disease and cultivation practice of shallot farmers in Bantul Coastal Area, Yogyakarta, Indonesia. J. Trop. Plant Pests Dis. 23(1): 23–30. https://doi.org/10.23960/jhptt.12323-30
Wong CKF, Saidi NB, Vadamalai G, Teh CY, & Zulperi D. 2019. Effect of bioformulations on the biocontrol efficacy, microbial viability and storage stability of a consortium of biocontrol agents against fusarium wilt of banana. J. Appl. Microbiol. 127(2): 544–555. https://doi.org/10.1111/jam.14310
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loqué D, Bowen BP, Firestone MK, Northen NR, & Brodie EL. 2018. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 3(4): 470–480. https://doi.org/10.1038/s41564-018-0129-3
Zhang Y, Tian C, Xiao J, Wei L, Tian Y, & Liang Z. 2020. Soil inoculation of Trichoderma asperellum M45a regulates rhizosphere microbes and triggers watermelon resistance to fusarium wilt. AMB Expr. 10: 189. https://doi.org/10.1186/s13568-020-01126-z
Zhao J, Liu J, Liang H, Huang J, Chen Z, Nie Y, Wang C, & Wang Y. 2018. Manipulation of the rhizosphere microbial community through application of a new bio-organic fertilizer improves watermelon quality and health. PLoS One. 13(2): e0192967. https://doi.org/10.1371/journal.pone.0192967