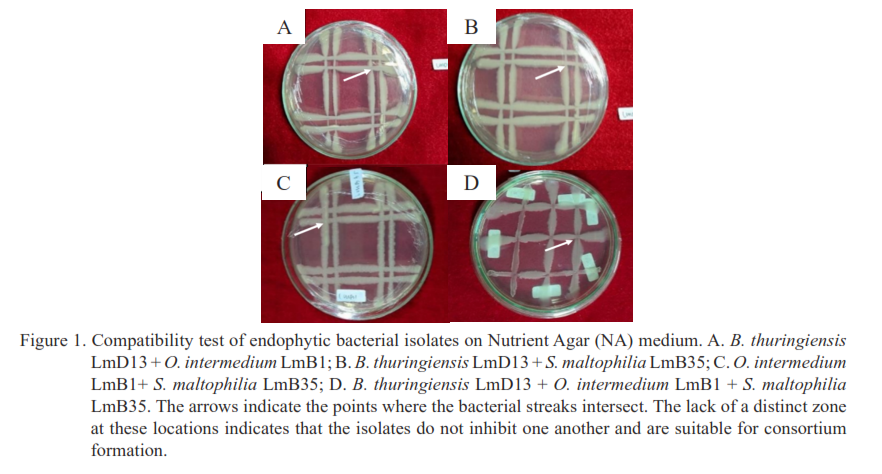
The potential in consortium of endophytic bacteria for controlling sheath blight by Rhizoctonia solani Kuhn in rice plants
Main Article Content
Abstract
Rhizoctonia solani Kühn is a pathogenic fungus that causes sheath blight disease in rice. One effective strategy for managing this disease is the use of biological control, particularly through consortia of endophytic bacteria. This study aimed to identify the most effective endophytic bacterial consortium for suppressing sheath blight severity while also enhancing rice growth and yield. A Completely Randomized Design (CRD) was aemployed with six treatments, three replications, and three experimental units per treatment. The treatments included four bacterial consortia composed of combinations of Bacillus thuringiensis LmD13, Ochrobactrum intermedium LmB1, and Stenotrophomonas maltophilia LmB35, along with positive and negative controls. The experiment involved treating rice seeds and soaking seedling roots with the bacterial consortia before transplanting. R. solani was inoculated onto the rice leaf sheaths 40 days after planting. The effectiveness of each consortium as a biocontrol agent was evaluated based on incubation period, disease incidence, disease severity, and the area under the disease progress curve (AUDPC). Their biostimulant potential was assessed through parameters related to seedling growth, plant development, and yield. Results indicated that the endophytic bacterial consortia effectively suppressed sheath blight and significantly improved rice growth and production. Notably, the consortium of B. thuringiensis LmD13, O. intermedium LmB1, and S. maltophilia LmB35 extended the incubation period to 35 days post-inoculation and reduced disease incidence, severity, and AUDPC to 22.22%, 0.29%, and 1.01, respectively. This consortium also enhanced rice yield, with fresh and dry grain weights reaching 72.78 g and 63.02 g, respectively, compared to the positive control. These findings suggest that this bacterial consortium holds strong potential as a biocontrol agent and yield enhancer in rice cultivation.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Al Banna MZ & Arifuddin W. 2021. Potensi bakteri asal bambu dalam memproduksi asam indol asetat (IAA) [The potential of bacteria from bamboo in producing indole acetic acid (IAA)]. Agrosainstek: Jurnal Ilmu dan Teknologi Pertanian. 5(1): 72–80. https://doi.org/10.33019/agrosainstek.v5i1.233
Aryantha INP & Lestari DP. 2004. The potency of IAA producing bacteria isolates on promotion the growth of mungbean sprout in hydroponic conditions. Jurnal Mikrobiologi Indonesia. 9(2): 43–46. https://doi.org/10.1016/S1049-9644(03)00160-6
Ashfaq B, Arshad HMI, Atiq M, Yousaf S, Saleem K, & Arshad A. 2021. Biochemical profiling of resistant phenotypes against Bipolaris oryzae causing brown spot disease in rice. Front. Agron. 3: 675895. https://doi. 10.3389/fagro.2021.675895
Ayaz M, Li CH, Ali Q, Zhao W, Chi YK, Shafiq M, Ali F, Yu XY, Yu Q, Zhao JT, Yu JW, Qi RD, & Huang WK. 2023. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules. 28(18): 6735. https://doi.org/10.3390/molecules28186735
Basu A, Chowdhurya S, Chaudhuri TR, & Kundu S. 2016. Differential behaviour of sheath blight pathogen Rhizoctonia solani in tolerant and susceptible rice varieties before and during infection. Plant Pathol. 65(8): 1333–1346. https://doi.org/10.1111/ppa.12502
Bakhat N, Vielba-Fernández A, Padilla-Roji I, Martínez-Cruz J, Polonio Á, Fernández-Ortuño D, Pérez-García A. 2023. Suppression of chitin-triggered immunity by plant fungal pathogens: A case study of the cucurbit powdery mildew fungus Podosphaera xanthii. J. Fungi. 9(7): 771. https://doi.org/10.3390/jof9070771
Carrión VJ, Perez-Jaramillo J, Cordovez V, Tracanna V, de Hollander M, Ruiz-Buck D, Mendes LW, van Ijcken WFJ, Gomez-Exposito R, Elsayed SS, Mohanraju P, Arifah A, der Oost JV, Paulson JN, Mendes R, Wezel GPV, Medema MH, Raaijmakers JM. 2019. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 366(6465): 606–612. https://doi.org/10.1126/science.aaw9285
Campbell CL & Madden LV. 1990. Introduction to Plant Disease Epidemiology. Wiley-Interscience. New York.
Denaya S, Yulianti R, Pambudi A, & Effendi Y. 2021. Novel microbial consortium formulation as plant growth promoting bacteria (PGPB) agent. IOP Conf. Ser.: Earth Environ. Sci. 637: 012030. https://doi.org/10.1088/1755-1315/637/1/012030
Desvani SD, Lestari IB, Wibowo HR, Supyani, Poromarto SH, & Hadiwiyono. 2018. Morphological characteristics and virulence of Rhizoctonia solani isolates collected from some rice production areas in some districts of Central Java. AIP Conf. Proc. 2014: 020068. https://doi.org/10.1063/1.5054472
Duncker KE, Holmes ZA, & You L. 2021. Engineered microbial consortia: Strategies and applications. Microb. Cell Fact. 20: 211. https://doi.org/10.1186/s12934-021-01699-9
El-Akhdar I, Elsakhawy T, & Abo-Koura HA. 2020. Alleviation of salt stress on wheat (Triticum aestivum L.) by plant growth promoting bacteria strains Bacillus halotolerans MSR-H4 and Lelliottia amnigena MSR-M49. J. Adv. Microbiol. 20(1): 44–58. https://doi.org/10.9734/jamb/2020/v20i130208
Fukagawa NK & Ziska LH. 2019. Rice: Importance for global nutrition. J. Nutr. Sci. Vitaminol (Tokyo). 65 (Supplement): S2–S3. https://doi.org/10.3177/jnsv.65.S2
Halimah D, Munif A, & Giyanto G. 2016. Potensi bakteri endofit Ochrobactrum intermedium-C939A31, Klebsiella oxytoca-C939A32, Bacillus subtilis-I308A32 asal tanaman kopi untuk mengendalikan nematoda luka akar Pratylenchus coffeae [The potential endophytic bacteria of Ochrobactrum intermedium- C939A31, Klebsiella oxytoca-C939A32, Bacillus subtilis-I308A32 of coffee plant for controlling root lesion nematode Pratylenchus coffeae]. Jurnal Fitopatologi Indonesia. 12(2): 62–68. https://doi.org/10.14692/jfi.12.2.62
Hallmann J. 2001. Plant interaction with endophytic bacteria. In: Jeger MJ & Spence NJ (Eds.). Biotic Interactions in Plant-Pathogen Associations. pp. 87–119. CABI Publising Wallingford. https://doi. 10.1079/9780851995120.0087
Huong NTM, Hoai PTT, Thao PTH, Huong TT, & Chinh VD. 2022. Growth stimulation, phosphate resolution, and resistance to fungal pathogens of some endogenous fungal strains in the rhizospheres of medicinal plants in Vietnam. Molecules. 27(16): 5051. https://doi.org/10.3390/molecules27165051
Imamuddin H, Dewi TK, Agustyani D, & Antonius S. 2015. Aktivitas Ochrobactrum sp. S79 L7T03, sebagai isolat yang bermanfaat untuk remediasi lingkungan tercemar dan agen pendukung pupuk organik hayati [Assessing the potential of Ochrobactrum sp. S79 L7T03 isolate for environmental remediation and as supporting agents of organic biofertilizer]. Pros. Sem. Nas. Masy. Biodiv. Indon. 1(2): 265–269. https://doi.org/10.13057/psnmbi/m010216
IRRI. 2002. Standard Evaluation System for Rice (SES). Internation Rice Research Institute. Manila. http://www.knowledgebank.irri.org/images/docs/rice-standard-evaluation-system.pdf
Jeyanthi V & Kanimozhi S. 2018. Plant growth promoting rhizobacteria (PGPR)-prospective and mechanisms: A review. J. Pure Appl. Microbiol. 12(2): 733–749. https://doi.org/10.22207/JPAM.12.2.34
Ju W, Liu L, Fang L, Cui Y, Duan C, & Wu H. 2019. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 167: 218–226. https://doi.org/10.1016/j.ecoenv.2018.10.016
Katsenios N, Andreou V, Sparangis P, Djordjevic N, Giannoglou M, Chanioti S, Kasimatis CN, Kakabouki I, Leonidakis D, Danalatos N, Katsaros G, & Efthimiadou A. 2022. Assessment of plant growth promoting bacteria strains on growth, yield and quality of sweet corn. Sci. Rep. 12: 11598. https://doi.org/10.1038/s41598-022-16044-2
Ke Y, Hui S, & Yuan M. 2017. Xanthomonas oryzae pv. oryzae inoculation and growth rate on rice by leaf clipping method. Bio-Protocol. 7(19): e2568. https://doi: 10.21769/BioProtoc.2568
Kesuma HI, Zuraidah, & Kamal S. 2016. Pengendalian penyakit blas yang disebabkan oleh cendawan patogen Pyricularia grisea dengan aplikasi bakteri pada tanaman padi (Oryza Sativa) var. Inpari 15 [Control of blast disease caused by pathogenic fungus Pyricularia grisea with bacterial application on rice plants (Oryza Sativa) var. Inpari 15]. Prosiding Seminar Nasional Biotik. 4(1): 286–291.
Khaeruni A, Rahim A, Syair, & Adriani. 2014. Induksi ketahanan terhadap penyakit hawar daun bakteri pada tanaman padi di lapangan menggunakan rizobakteri indigenos [Induced resistance to bacterial leaf blight disease in rice field by indigenous rhizobacteria]. J. Trop. Plant Pests Dis. 14(1): 57–63. https://doi.org/10.23960/j.hptt.11457-63
Klement Z, Rudolph K, & Sand DC. 1990. Methods in Phytobacteriology. Akadémiai Kiadó. Budafest.
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, & Raaijmakers JM. 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 332(6033): 1097–1100. https://doi.10.1126/science.1203980
Milati LN & Nuryanto B. 2019. Periode kritis pertumbuhan tanaman padi terhadap infeksi penyakit hawar pelepah dan pengaruhnya terhadap hasil gabah [Critical period of rice plant growth against sheath blight infection and the impact on yield]. Jurnal Penelitian Pertanian Tanaman Pangan. 3(2): 61.
Molla KA, Karmakar S, Molla J, Bajaj P, Varshney RK, Datta SK, & Datta K. 2020. Understanding sheath blight resistance in rice: The road behind and the road ahead. Plant Biotechnol. J. 18(4): 895–915. https://doi.org/10.1111/pbi.13312
Morales-Cedeño LR, Orozco-Mosqueda Ma.DC, Loeza-Lara PD, Parra-Cota FI, Santos-Villalobos SDL, & Santoyo G. 2021. Plant growth-promoting bacterial endophytes as biocontrol agents of pre-and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 242: 126612. https://doi.org/10.1016/j.micres.2020.126612
Motoyama T. 2020. Secondary metabolites of the rice blast fungus Pyricularia oryzae: Biosynthesis and biological function. Int. J. Mol. Sci. 21(22): 8698. https://doi.10.3390/ijms21228698
Nunes PSO, Lacerda-Junior GV, Mascarin GM, Guimarães RA, Medeiros FHV, Arthurs S, & Bettiol W. 2024. Microbial consortia of biological products: Do they have a future?. BioControl. 188: 105439. https://doi.org/10.1016/j.biocontrol.2024.105439
Olanrewaju OS, Glick BR, & Babalola OO. 2017. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 33: 197. https://doi. 10.1007/s11274-017-2364-9
Oliva R, Ji C, Atienza-Grande G, Huguet-Tapia, Perez-Quintero A, Li T, Eom JS, Li C, Nguyen H, Liu B, Auguy F, Sciallano C, Lu VT, Dossa GS, Cunnac S, Schmidt SM, Slamet-Loedin IH, Cruz CV, Szurek B, Frommer WB, White FF, & Yang B. 2019. Broadspectrum resistance to bacterial blight in rice using genome editing. Nature Biotechnology. 37(11): 1344–1350. https://doi.org/10.1038/s41587-019-0267-z
Ou SH. 1985. Rice Diseases. 2nd Edition, Commonwealth Mycological Institute. Kew.
Panwar M, Tewari R, & Nayyar H. 2014. Microbial consortium of plant growth-promoting rhizobacteria improves the performance of plants growing in stressed soils: An Overview. In: Khan M, Zaidi A, Musarrat J. (Eds.). Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology. pp. 257–285. Springer Cham. Switzerland. https://doi.org/10.1007/978-3-319-08216-5_11
Prasad M, Srinivasan R, Chaudhary M, Choudhary M, & Jat LK. 2019. Chapter Seven - Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture: Perspectives and Challenges. In: Singh AK, Kumar A, & Singh PK (Eds.). PGPR Amelioration in Sustainable Agriculture. Food Security and Environmental Management. pp. 129–157. Woodhead Publishing. United Kingdom. https://doi.org/10.1016/B978-0-12-815879-1.00007-0
Rahma H, Nurbailis, & Kristina N. 2019. Characterization and potential of plant growth-promoting rhizobacteria on rice seedling growth and the effect on Xanthomonas oryzae pv. oryzae. Biodiversitas. 20(12): 3654–3661. https://doi.org/10.13057/biodiv/d201226
Rahma H, Nurbailis, Busniah M, Kristina N, & Larasati Y. 2022. The potential of endophytic bacteria to suppress bacterial leaf blight in rice plants. Biodiversitas. 23(2): 775–782. https://doi.10.13057/biodiv/d230223
Rahma H, Trizelia, Martinius, Liswarni Y, Rusli R, Rozen N, Armelia L, Putri, & Qalbina F. 2024. Characterization of plant growth promoting bacteria (PGPB) as biocontrol agents against plant pathogenic fungi in vitro. Intl. J. Agric. Biol. 32(3): 201–209. https://doi.10.17957/IJAB/15.2193
Rao TB, Chopperla R, Prathi NB, Balakrishnan M, Prakasam V, Laha GS, Balachandran SM, & Mangrauthia SK. 2020. A comprehensive gene expression profile of pectin degradation enzymes reveals the molecular events during cell wall degradation and pathogenesis of rice sheath blight pathogen Rhizoctonia solani AG1-IA. J Fungi. 6(2): 71. https://doi. org/10.3390/jof6020071
Rat A, Naranjo HD, Krigas N, Grigoriadou K, Maloupa E, Alonso AV, Schneider C, Papageorgiou VP, Assimopoulou AN, Tsafantakis N, Fokialakis N, & Willems A. 2021. Endophytic bacteria from the roots of the medicinal plant Alkanna tinctoria Tausch (Boraginaceae): Exploration of plant growth promoting properties and potential role in the production of plant secondary metabolites. Front. Microbiol. 12: 633488. https://doi.org/10.3389/fmicb.2021.633488
Sandy G, Ratih S, Suharjo R, & Akin HM. 2019. Pengaruh Trichoderma sp. sebagai agen peningkatan ketahanan tanaman padi terhadap penyakit hawar daun [The effect of Trichoderma sp. as an agent for increasing rice plant resistance to leaf blight disease]. J. Agrotek Tropika. 7(3): 423–432. https://doi.org/10.23960/jat.v7i3.3546
Santoyo, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR. 2016. Plant growthpromoting bacterial endophytes. Microbiological Research. 183: 92–99. https://doi.org/10.1016/j.micres.2015.11.008
Santoyo G, Guzmán-Guzmán P, Parra-Cota FI, Santos-Villalobos SD, Orozco-Mosqueda MdC, & Glick BR. 2021. Plant growth stimulation by microbial consortia. Agronomy. 11(2): 219. https://doi.org/10.3390/agronomy11020219
Sarma BK, Yadav SK, Singh S, & Singh HB. 2015. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 87: 25–33. https://doi.org/10.1016/j.soilbio.2015.04.001
Sattari A, Nohtani H, Azhdarpoor NA, Ghadami M, Madahinasab MO, Moradi K, & Noorozi MO. 2015. Brown spot resistance in rice: A Review. IJSRST. 1(5): 175–179.
Sen S, Chakrabortya R, & Kalita P. 2020. Rice - not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Tech. 97: 265–285. https://doi.org/10.1016/j.tifs.2020.01.022
Senapati M, Tiwari A, Sharma N, Chandra P, Bashyal BM, Ellur RK, Bhowmick PK, Bollinedi H, Vinod KK, Singh AK, & Krishnan SG. 2022. Rhizoctonia solani Kühn pathophysiology: Status and prospects of sheath blight disease management in rice. Front. Plant Sci. 13: 881116. https://doi.org/10.3389/fpls.2022.881116
Sharma T, Kumar N, & Rai N. 2012. Isolation, screening and characterization of PGPR isolates from rhizosphere of rice plants in Kashipur region (Tarai region). Biotechnol. Int. 5: 69–84.
Singh P, Mazumdar P, Harikrishna JA, & Babu S. 2019. Sheath blight of rice: A review and identification of priorities for future research. Planta. 250: 1387–1407. https://doi.10.1007/s00425-019-03246-8
Singh A, Yadav VK, Chundawat RS, Soltane R, Awwad NS, Ibrahium HA, Yadav KK, & Vicas SI. 2023. Enhancing plant growth promoting rhizobacterial activities through consortium exposure: A review. Front. Bioeng. Biotechnol. 11: 1099999. https://doi.org/10.3389/fbioe.2023.1099999
Solanki MK, Yandigeri MS, Kumar S, Singh RK, & Srivastava AK. 2019. Co-inoculation of different antagonists can enhance the biocontrol activity against Rhizoctonia solani in tomato. Antonie van Leeuwenhoek. 112: 1633–1644. https://doi.org/10.1007/s10482-019-01290-8
Srinivasan K & Mathivanan N. 2011. Plant growth promoting microbial consortia mediated classical biocontrol of sunflower necrosis virus disease. J. Biopest. 4(1): 65–72. https://doi.org/10.57182/jbiopestic.4.1.65-72
Stockwell VO, Johnson KB, Sugar D, & Loper JE. 2011. Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology. 101(1): 113–123. https://doi.org/10.1094/PHYTO-03-10-0098
Suryadi Y, Samudra IM, Priyatno TP, Susilowati DN, Lestari P, & Sutoro. 2015. Aktivitas anticendawan Bacillus cereus 11UJ terhadap Rhizoctonia solani dan Pyricularia oryzae. [Antifungal activity of Bacillus cereus 11UJ against Rhizoctonia solani and Pyricularia oryzae]. Jurnal Fitopatologi Indonesia. 11(2): 35–35. https://doi.org/10.14692/jfi.11.2.35
Sen S, Chakrabortya R, & Kalita P. 2020. Rice - not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Techno. 97: 265–285. https://doi.org/10.1016/j.tifs.2020.01.022
Shahzad R, Waqas M, & Khan AL. 2017. Indoleacetic acid production and plant growth promoting potential of bacterial endophytes isolated from rice (Oryza sativa L.) seeds. Biologia Futura. 68(2): 175–186. https://doi. 10.1556/018.68.2017.2.5
Sivalingam PN, Vishwakarma SN, & Singh US. 2006. Role of seed-borne inoculum of Rhizoctonia solani in sheath blight of rice. Indian Phytopath. 59(4): 445–452.
Sutrawati M , Ganefianti DW, Sipriyadi S ,Wibowo RH, Agustin Z, Listihani, & Selangga DGW. 2021. Disease incidence and molecular diversity of tungro virus on rice (Oryza sativa) in Bengkulu, Indonesia. IJAT. 17(5): 1973–1984.
Tiwari S, Prasad V, Chauhan PS, & Lata C. 2017. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant. Sci. 8: 1510. https://doi.org/10.3389/fpls.2017.01510
Ullah A, Nisar M, Ali H, Hazrat A, Hayat K, Keerio AA, Ihsan M, Laiq M, Ullah S, Fahad S, Khan AH, Akbar A, & Yang X. 2019. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 103: 7385–7397. https://doi.org/10.1007/s00253-019-10045-4
van Loon LC. 2007. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 119: 243–254. https://doi.org/10.1007/s10658-007-9165-1
Whipps. 2001. Microbial interactions and biocontrol in the rhizosphere. J Exp Botany. 52: 487–511.https://doi.org/10.1093/jexbot/52.suppl_1.487
Wu X, Liu H, Lian B, Jiang X, Chen C, Tang T, Ding X, Hu J, Zhao S, Zhang S, & Wu J. 2023. Genome-wide analysis of epigenetic and transcriptional changes in the pathogenesis of RGSV in rice. Front. Plant Sci. 13: 1090794. https://doi.org/10.3389/fpls.2022.1090794
Yanti Y, Hamid H, Reflin, Warnita, & Habazar H. 2020. The ability of indigenous Bacillus spp. consortia to control the anthracnose disease (Colletrotricum capsici) and increase the growth of chili plants. Biodiversitas. 21(1): 179–186. https://doi.10.13057/biodiv/d210123
Yuniawati R & Akhdiya A. 2021. Karakterisasi isolat bakteri endofit nilam (Pogostemon cablin B.) sebagai kandidat biostimulan pertumbuhan tanaman [Characterization of patchouli endophytic bacterial isolates (Pogostemon cablin B.) as plant growth biostimulants candidates]. Bul. Plasma Nutfah. 27(1): 21–28.