Effectiveness of bionematicide from Purpureocillium lilacinum in controlling root-knot nematodes (Meloidogyne spp.)
Main Article Content
Abstract
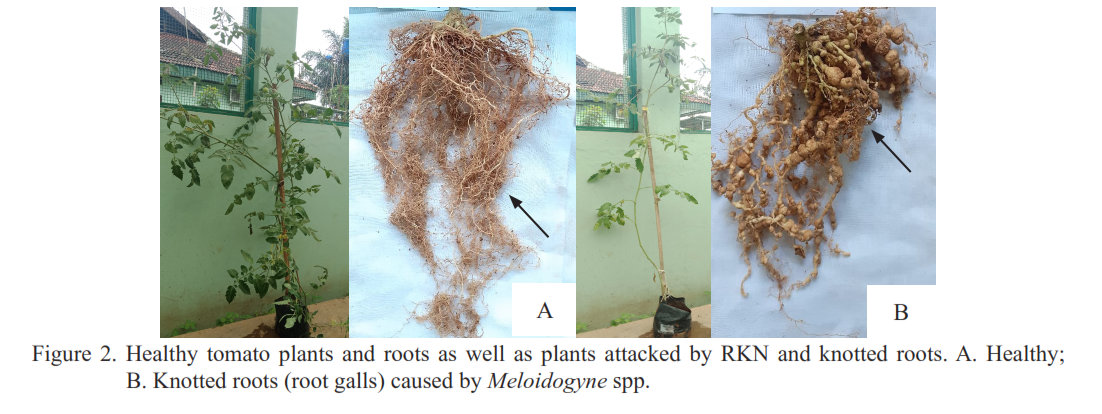
This research aimed to study the efficacy of the fungus Purpureocillium lilacinum as a bionematicide to control root-knot nematodes (RKN). Two steps of experiments were carried out in this study. The first experiment involved the application of various levels of bionematicide doses to control RKN on tomato plants. The second experiment tested the application of bionematicide (both as a single application and in combination with bromelain compost) to control RKN on guava cv. Kristal. A carbofuran nematicide was applied following the company’s recommendation in this second experiment for comparison. The results of the first trial showed that the application of P. lilacinum bionematicide at doses ranging from 20–40 g per plant or 7–13 g per kg of soil was effective in reducing the J-2 RKN population in the soil and roots, as well as mitigating damage to plant roots. In the second experiment, it was shown that the application of P. lilacinum bionematicide, either alone or mixed with bromelain compost, was more effective than the application of carbofuran nematicide in reducing the J-2 RKN population in the soil and roots, as well as in minimizing root damage to guava seedlings. Additionally, the application of bionematicides mixed with compost proved more effective than their single application in reducing plant root damage. Furthermore, apart from being able to control nematode populations and plant damage, P. lilacinum bionematicide could stimulate plant growth.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Abbas H, Javed N, Khan SA, ul Haq I, Ali MA, & Safdar A. 2011. Integration of bioagent and bioproduct for the management of Root-Knot Nematodes, Meloidogyne incognita on eggplant. Journal of Biology, Agriculture and Healthcare. 1(4): 31–36.
Abd-Elgawad MMM & Askary TH 2018. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt J. Biol. Pest Control. (28)74: 1–12. https://doi.org/10.1186/s41938-018-0080-x
Barker KR. 1985. Nematode extraction and bioassays. In: Barker KR, Carter CC, & Sasser JN (Eds.). An Advanced Treatise on Meloidogyne: Vol II Methodology. International Meloidogyne Project. North Caroline, USA. P. 20–35.
Baron NC, Souza Pollo A, & Rigobelo EC. 2020. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ. 8: e9005. https://doi.org/10.7717/peerj.9005
Brand D, Soccol CR, Sabu A, & Roussos S. 2009. Production of fungal biological control agents through solid state fermentation: A case study on Paecilomyces lilacinus against root-knot nematodes. Micol. Apl. Int. 22(1): 31–48.
Dawabah AAM, Al-Yahya FA, & Lafi HA. 2019. Integrated management of plant parasitic nematodes on guava and fig trees under tropical field conditions. Egypt J. Biol. Pest Control. 29(29): 1–9. https://doi.org/10.1186/s41938-019-0133-9
El-Ashry RM, Ali MAS, Elsobki AEA, & Aioub AAA. 2021. Integrated management of Meloidogyne incognita on tomato using combinations of abamectin, Purpureocillium lilacinum, rhizobacteria, and botanicals compared with nematicide. Egypt J. Biol. Pest Control. 31(93): 1–10. https://doi.org/10.1186/s41938-021-00438-x
Fiandani A, Swibawa IG, Fitriana Y, & Purnomo. 2021. Pengaruh dosis bionematisida Purpureocillium lilacinum (Syn. Paecilomyces lilacinus) isolat B01TG berbahan pembawa limbah pertanian terhadap kefektifannya dalam mengendalikan Meloidogyne spp. [The effect of bionematicide fungi Purpureocillium lilacinum (syn. Paecilomyces lilacinus) B01TG isolate with agricultural waste carrier materials on their effectiveness within control Meloidogyne spp.] Jurnal Agrotek tropika. 9(2): 189–197. https://doi.org/10.23960/jat.v9i2.5016
Grabau ZJ, Mauldin MD, Habteweld A, & Carter ET. 2020. Nematicide efficacy at managing Meloidogyne arenaria and non-target effects on free-living nematodes in peanut production. J. Nematol. 52(1): e2020-28. https://doi.org/10.21307/jofnem-2020-028
Grace GN, Shivananda TN, Rao MS, & Umamaheswari R. 2019. Management of nematodes using liquid formulations of Purpureocillium lilacinum on tuberose. JEZS. 7(1): 720–724.
Gyawali K. 2018. Pesticide uses and its effects on publict health and environment. Journal of Health Promotion. 6: 28–36. https://doi.org/10.3126/jhp.v6i0.21801
Hara AH & Kaya HK. 1982. Effect of selected insecticides and nematicides on the in vitro development of the entomogenous nematode Neoaplectana carpocapsae. J. Nematol. 14(4): 486–491.
Hooper DJ, Hallman J, & Subbotin SA. 2005. Methods for extraction, processing and detection of plant and soil nematodes. In: Luc M, Sikora RA, Bridge J (Eds.). Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. Pp. 53–86. Second Ed. CABI Publishing. Wallingford.
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Herder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WML, & Perry RN. 2013. Top 10 plant-parasitic nematodes in moleculer plant pathology. Molecular Plant Pathology. 14(9): 946–961. https://doi.org/10.1111/mpp.12057
Kurniawati F, Nursipa NT, & Munif A. 2020. Nematoda puru akar pada seledri (Apium graviolens L.) dan pengendaliannya menggunakan bakteri endofit secara in vitro. [Rootknot nematodes in celery (Apium graveolens L.) and its in vitro control using endophytic bacteria]. Agrovigor. 13(1): 70–81. https://doi.org/10.21107/agrovigor.v13i1.6304
Lamovšek J, Urek G, & Trdan S. 2013. Biological control of root-knot nematodes (Meloidogyne spp.): Microbes against the pests. Acta Agric. Slov. 101(2): 263–275. https://doi.org/10.2478/acas-2013-0022
Mirsam H & Kurniawati F. 2018. Laporan pertama di Sulawesi Selatan: Karakter morfologi dan molekuler nematoda puru akar yang berasosiasi dengan akar padi di Kabupaten Wajo Sulawesi Selatan [First report in south Sulawesi: morphological and molecular characters of root knot nematodes associated with rice root in Wajo, South Sulawesi]. JPTI. 22(1): 58–65. https://doi.org/10.22146/jpti.33108
Mutala’liah M, Indarti S, & Wibowo A. 2019. Short Communication: The prevalence and species of root-knot nematode which infect on potato seed in Central Java, Indonesia. Biodiversitas. 20(1): 11–16. https://doi.org/10.13057/biodiv/d200102
Nabilah, Swibawa IG, Suharjo R, & Fitriana Y. 2021. Diversity and abundance of nematodes in guava (Psidium guajava L.) cultivation in lampung. J. Trop. Plant Pests Dis. 21(2): 134–143. https://doi.org/10.23960/jhptt.221134-143
Nurjayadi MY, Munif A, & Suastika G. 2015. Identifikasi nematoda puru akar, Meloidogyne graminicola pada tanaman padi di Jawa Barat [Identification of Root Knot Nematodes, Meloidogyne graminicola, on rice in West Java]. Jurnal Fitopatol. Indones. 11(4): 113–130. https://doi.org/10.14692/jfi.11.4.113
R Core Team. 2021. R: A languane and environment for statistical computing. R Fundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Sharma P & Pandey R. 2009. Biological control of root- knot nematode; Meloiodogyne incognita in the medicinal plant; Withania somnifera and the effect of biocontrol agents on plant growt. Afr. J. Agric. Res. 4(6): 564–567.
Singh S, Pandey RK, & Goswami BK. 2013. Bio-control activity of Purpureocillium lilacinum strains in managing root-knot disease of tomato caused by Meloidogyne incognita. Biocontrol Sci. Techn. 23(12): 1469–1489. https://doi.org/10.1080/09583157.2013.840770
Seenivasan N. 2017. Combined application of Pseudomonas fluorescens and Purpureocillium lilacinum liquid formulations to manage Globodera spp. on potato. J. Crop Prot. 6(4): 529–537.
Sundararaju P & Cannayane I. 2002. Production of nematode egg parasitic fungus, Paecilomyces lilacinus, on banana wastes and certain plant leaves. Indian J. Nematol. 32(2): 188–189.
Supramana & Suastika G. 2012. Spesies nematoda puru akar (Meloidogyne spp.) yang berasosiasi dengan penyakit umbi bercabang pada wortel: penyakit baru di Indonesia [Root knot nematode species, Meloidogyne spp., which associating with the branched tuber disease of carrot: a new disease in Indonesia]. Jurnal Ilmu Pertanian Indonesia. 17(2): 108–112. https://journal.ipb.ac.id/index.php/JIPI/article/view/8324
Swibawa IG, Fitriana Y, Solikhin, Suharjo R, Susilo FX, Rani E, Haryani MS, & Wardana RA 2020. Morpho-molecular identification and pathogenicity test on fungal parasites of guava root-knot nematode eggs in Lampung, Indonesia. Biodiversitas. 21(3): 1108–1115. https://doi.org/10.13057/biodiv/d210334
Watson TT & Desaeger DA. 2019. Evaluation of non-fumigant chemical and biological nematicides for strowberry production in Florida. Crop Prot. 117: 100–107. https://doi.org/10.1016/j.cropro.2018.11.019
Zhan J, Qin Y, Gao K, Fan Z, Wang L, Xing R, Liu S, & Li P. 2021. Efficacy of a chitin-based water-soluble derivative in inducing Purpureocillium lilacinum against nematode disease (Meloidogyne incognita). Int. J. Mol. Sci. 22(13): 6870. https://doi.org/10.3390/ijms22136870
Yankova V, Markova D, Naidenov M, & Arnaoudov B. 2014. Management of root-knot nematodes (Meloidogyne spp.) in greenhouse cucumbers using microbial products. TURKJANS. 2: 1569–1573.
Zeck WM. 1971. A rating scheme for field evaluation of root-knot nematode infestation. Pflanzenschutz-Nachr Bayer. 24(1): 141–144.