Fusarium keratoplasticum TKKS-1: A potential native entomopathogenic fungus to control Armyworm, Spodoptera litura Fabricus (Lepidoptera: Noctuidae), on mustard plants
Main Article Content
Abstract
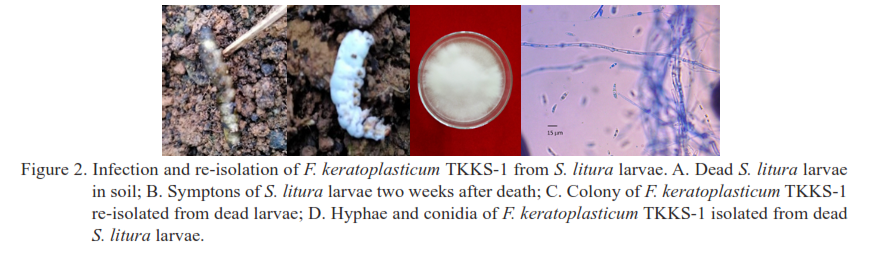
The use of entomopathogen (insect pathogen) is one of the effective strategies for managing insect pests. This study aimed to evaluate the efficacy of the entomopathogenic fungus Fusarium keratoplasticum against Spodoptera litura larvae under laboratory conditions and to assess its potential in controlling infestations on mustard plants. The pathogenicity of the fungal isolate was tested against third-instar S. litura larvae at a concentration of 1 × 107 conidia/mL. Both fungal isolates caused 100% larval mortality, however, F. keratoplasticum acted more rapidly than Beauveria bassiana, reaching 100% mortality within 6 days, compared to 9 days for B. bassiana. The virulence of F. keratoplasticum was further evaluated using a Simple Completely Randomized Block Design (SCBD) consisting of five treatments with different conidial concentrations (1 × 106, 2 × 106, 4 × 106, 8 × 106, and 1 × 107 conidia/mL) and an untreated control. A commercial B. bassiana formulation (1 × 107 conidia/mL) served as a comparison. The application technique involved direct exposure of S. litura larvae to the fungal suspensions. The LC50 value of F. keratoplasticum was 2.74 × 106 conidial/mL, while the LT50 value at 1 × 107 conidia/mL was 2.96 days, significantly shorter than that of B. bassiana (LT50 = 3.63 days). Under semi-field conditions, F. keratoplasticum demonstrated superior effectiveness in controlling S. litura larvae on mustard plants, achieving complete mortality more rapidly than under laboratory conditions and outperforming B. bassiana. These findings indicate that F. keratoplasticum has strong potential to be developed as a biopesticide.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Abbott WS. 1925. A method of computing the effectiveness of an insecticide. J Econ Entomol. 10(2): 265–267. https://doi.org/10.1093/jee/18.2.265a
Abbas C, Xiang D, Wei H, Liu S, Yi G, Lyu S, Guo L, & Li C. 2020. Predicting virulence of Fusarium oxysporum f. sp. Cubense based the production of Mycotoxin using a linear regression model. Toxins (Basel). 12(4): 254. https://doi.org/10.33990/toxins12040254
Acheampong MA, Coombes CA, Moore SD, & Hill MP. 2020. Temperature tolerance and humidity requirements of select entomopathogenic fungal isolates for future use in citrus IPM programmes. J. Invertebr. Pathol. 174: 107436. https://doi.org/https://doi.org/10.1016/j.jip.2020.107436
Afandhi A, Rachmawati R, Syib’li MA, & Zain HAU. 2023. Performance and virulence of the entomopathogenic fungi Beauveria bassiana grown in media derived from biodegradable agricultural wastes enriched with cricket powder. AGRIVITA J. Agric. Sci. 45(2): 261–270. http://dx.doi.org/10.17503/agrivita.v45i2.4113
Bamisile BS, Akutse KS, Siddiqui JA, & Xu Y. 2021. Model application of entomopathogenic fungi as alternatives to chemical pesticides: Prospects, challenges, and insights for next-generation sustainable agriculture. Front. Plant Sci. 12: 741804. https://doi.org/10.3389/fpls.2021.741804
BPTD. 2011. Strategi Pengendalian Hama Penyakit Tanaman Tembakau. BPTD PTP Nusantara II. Medan.
EFSA Panel on Plant Health (PLH), Bragard C, Dehnen-Schmutz K, Di Serio F, Gonthier P, Jacques MA, Miret JAJ, Justesen AF, Magnusson CS, Milonas P, Navas-Cortes JA, Parnell S, Potting R, Reignault PL, Thulke HH, Van der Werf W, Civera AV, Yuen J, Zappalà L, Malumphy C, Czwienczek E, & MacLeod A. 2019. Pest categorisation of Spodoptera litura. EFSA Journal. 17(7): e05765. https://doi.org/10.2903/j.efsa.2019.5765
Chehri K. 2017. Molecular identification of entomopathogenic Fusarium species associated with Tribolium species in stored grains. J Invertebr. Pathol. 144: 1–6. https://doi.org/https://doi.org/10.1016/j.jip.2017.01.003
Chiewchanvit S, Chongkae S, Mahanupab P, Nosanchuk J, Pornsuwan S, Vanittanakom N, & Youngchim S. 2017. Melanization of Fusarium keratoplasticum (F. solani species complex) during disseminated Fusariosis in a patient with acute leukemia. Mycopathologia. 182: 879–885. https://doi.org/doi: 10.1007/s11046-017-0156-2
da Silva Santos AC, Diniz AG, Tiago PV, & de Oliveira NT. 2020. Entomopathogenic Fusarium species: A review of their potential for the biological control of insects, implications and prospects. Fungal Biol. Rev. 34(1): 41–57. https://doi.org/10.1016/j.fbr.2019.12.002
Guo Z, Pfohl K, Karlovsky P, Dehne HW, & Altincicek B. 2018. Dissemination of Fusarium proliferatum by mealworm beetle Tenebrio molitor. PloS One. 13(9): e0204602. https://doi.org/10.1371/journal.pone.0204602
Han Z, Xie Y, Xue J, & Fan J. 2010. Effect of multi-generation culture on virulence of Lecanicillium lecanii in different media. Wei Sheng Wu Xue Bao. 50(2): 211–221.
Isobe M, Kato S, Suzuki M, Nannya Y, Takahashi S, & Konuma S. 2024. Disseminated Fusarium keratoplasticum infection with myocardial involvement in an adult cord blood transplant recipient. Mycopathologia. 189: 95. https://doi.org/10.1007/s11046-024-00900-y
James JE, Santhanam J, Zakaria L, Rusli NM, Bakar MA, Suetrong S, Sakayaroj J, Razak MFA, Lamping E, & Cannon RD. 2022. Morphology, phenotype, and molecular identification of clinical and environmental Fusarium solani species complex isolates from Malaysia. J. Fungi. 8(8): 845. https://doi.org/10.3390/jof8080845
Mantzoukas S, Kitsiou F, Lagogiannis I, & Eliopoulos PA. 2022. Potential use of Fusarium isolates as biological control agents: Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) case study. Appl. Sci. 12(17): 8919. https://doi.org/10.3390/app12178918
Mishra S, Kumar P, & Malik A. 2015. Effect of temperature and humidity on pathogenicity of native Beauveria bassiana isolate against Musca domestica L. J. Parasit Dis. 39(4): 697–704. https://doi.org/10.1007/s12639-013-0408-0
Praneetha TP, Masih SA, Addesso R, Maxton A, & Sofo A. 2025. Brassicaceae isothiocyanate-mediated alleviation of soil-borne diseases. Plants. 14: 1200. https://doi.org/10.3390/plants14081200
Pelizza SA, Stenglein SA, Cabello MN, Dinolfo MI, & Lange CE. 2011. First record of Fusarium verticillioides as an entomopathogenic fungus of grasshoppers. J. Insect Sci. 11(1): 70. https://doi.org/10.1673/031.011.7001
Sahid A & Kusumaningtyas P. 2023. Characterization and virulence of two indigenous entomopathogenic fungal isolates from decayed oil palm empty fruit bunches against Spodoptera litura (Lepidoptera: Noctuidae). Biodiversitas. 24(2): 1192–1199. https://doi.org/10.13057/biodiv/d240260
Saleem AR & Ibrahim RA. 2019. Assessment of the virulence and proteolytic activity of three native entomopathogenic fungi against the larvae of Oryctes agamemnon (Burmeister) (Coleoptera: Scarabaeidae). Egypt J. Biol. Pest Control. 29: 21. https://doi.org/10.1186/s41938-019-0120-1
Santos ACdS, Diniz AG, Tiago PV, & Oliveira NTd. 2020. Entomopathogenic Fusarium species: A review of their potential for the biological control of insects, implications and prospects. Fungal Biol. Rev. 34(1): 41–57. https://doi.org/https://doi.org/10.1016/j.fbr.2019.12.002
Saputro TB, Prayogo Y, Rohman FL, & Alami NH. 2019. The virulence improvement of Beauveria bassiana in infecting Cylas formicarius modulated by various chitin based compounds. Biodiversitas. 20(9): 2486–2493. https://doi.org/10.13057/biodiv/d200909
Sharma L & Marques G. 2018. Fusarium, an entomopathogen—a myth or reality?. Pathogens. 7(4): 93. https://doi.org/10.3390/pathogens7040093
Short DPG, O’Donnell K, Zhang N, Juba JH, & Geiser DM. 2011. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J. Clin. Microbiol. 49(12): 4264–4272. https://doi.org/10.1128/jcm.05468-11
Song Y, Cang X, He W, Zhang H, & Wu K. 2024. Migration activity of Spodoptera litura (Lepidoptera: Noctuidae) between China and the South-Southeast Asian region. Insects. 15(5): 335. https://doi.org/10.3390/insects15050335
Szaliński M, Zgryźniak A, Rubisz I, Gajdzis M, Kaczmarek R, & Przeździecka-Dołyk J. 2021. Fusarium keratitis—Review of current treatment possibilities. J. Clin. Med. 10(23): 5468. https://doi.org/10.3390/jcm10235468