Hemolysis and hypersensitive tests ease culture collection management of antagonistic bacteria
Main Article Content
Abstract
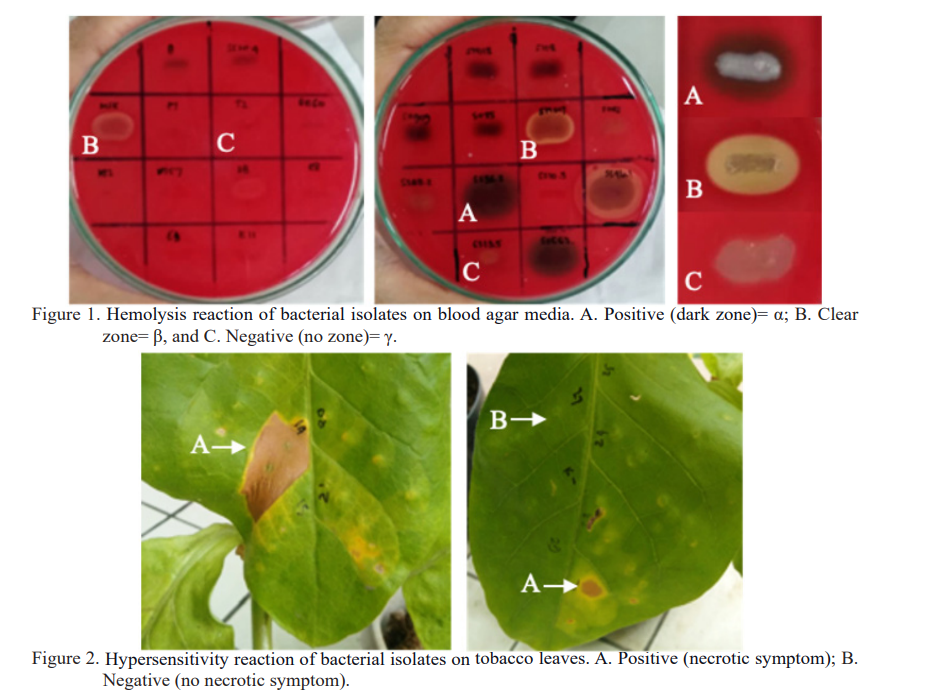
A biosafety assessment is a crucial first step in the management of microbial culture collection to screen and determine unexpected potential plant and human pathogenic bacteria. It is common to collect and store as many fresh culture collections from natural resources before being further evaluated for antagonist bacteria. As a result, a bulk of isolates must be preserved which required more effort and budget. Safety evaluations based on the hemolysis and hypersensitive reactions offer simple tests to ease culture collection management of antagonist bacteria. The study aimed to evaluate the safety of bacterial culture collection for their hemolysis and hypersensitive reactions. Ninety-five isolates of rhizosphere and endophytic bacterial isolates from the culture collections of the Department of Plant Protection-IPB University, Indonesian Industrial and Beverages Crops Research Institute, and Indonesian Spice and Medicinal Crops Research Institute, were evaluated their safety using the hemolysis and hypersensitive tests. The hemolysis test was conducted using blood agar media, from which isolates with a negative (?) reaction were then tested for the hypersensitivity reaction on tobacco leaves. Bacterial isolates passed from both hemolysis and hypersensitivity tests were then preserved by the lyophilization method for long-term storage of culture collection. Based on the hemolysis test, 68 out of 95 bacterial isolates (71.57%) were found to be positive (? or ?) reactions. The hypersensitive test showed that 22 of 27 negative hemolysis isolates did not trigger hypersensitivity reactions in tobacco leaves, therefore, they were preserved by lyophilization. The study indicated that a high number of bacterial isolates in the present collection, 68 positive hemolysis, and 5 hypersensitive, need to be re-evaluated due to their safety concerns. The present study highlights the importance of biosafety tests performed in an early stage before the to permanent collection of antagonist isolates.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Akhdiya A, Wartono, Sulaeman E, & Samudra IM. 2018. Karakterisasi bakteri pendegradasi profenofos [Characterization of profenofos degrading bacteria]. Jurnal AgroBiogen. 14(1): 37–46.
Amaria W, Kasim NN, & Munif A. 2019. Kelimpahan populasi bakteri filosfer, rizosfer, dan endofit tanaman kemiri (Reutealis trisperma (Blanco) Airy Shaw) serta potensinya sebagai agens biocontrol [Abundance population of phyllosphere, rhizosphere, and endophytes bacteria from philippine tung (Reutealis trisperma (Blanco) Airy Shaw) and its potential as biocontrol agents]. Journal TABARO. 3(1): 305–317. https://doi.org/10.35914/tabaro.v3i1.200
Berg G, Eberl L, & Hartmann A. 2005. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7(11): 1673–1685. https://doi.org/10.1111/j.1462-2920.2005.00891.x
Buxton R. 2016. Blood Agar Plates and Hemolysis Protocol. American Society for Microbiology.
Díaz-Rodríguez AM, Gastelum LAS, Pablos CMF, Parra-Cota FI, Santoyo G, Puente ML, Bhattacharya D, Mukherjee J, & Santos-Villalobos Sdl. 2021. The current and future role of microbial culture collections in food security worldwide. Front. Sustain. Food Syst. 4: 614739. https://doi.org/10.3389/fsufs.2020.614739
Fletcher J, Leach JE, Eversole K, & Tauxe R. 2013. Human pathogens on plants: Designing a multidisciplinary strategy for research. Phytopathology. 103(4): 306–315. https://doi.org/10.1094/PHYTO-09-12-0236-IA
Gilligan PH. 2013. Identification of pathogens by classical clinical tests. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (Eds.). The Prokaryotes-Human Microbiology. Springer-Verlag Berlin Heidelberg. pp. 57–89. https://doi.org/10.1007/978-3-642301445_90
Heath MC. 2000. Hypersensitive response-related death. Plant Mol. Biol. 44: 321–334. https://doi.org/10.1023/A:1026592509060
Klement Z & Goodman RN. 1967. The role of the living bacterial cell and induction time in hypersensitive reaction of tobacco plants. Phytopathology. 57: 322–323.
Kupletskaya MB, & Netrusov AI. 2011. Viability of lyophilized microorganisms after 50-year storage. Microbiology. 80: 850–853. https://doi.org/10.1134/S0026261711060129
Leahy J, Mendelsohn M, Kough J, Jones R, & Berckes N. 2014. Biopesticide oversight and registration at the U.S. Environmental Protection Agency. In: Gross AD, Coats JR, Duke SO, & Seiber JN (Eds.). Biopesticides: State of the Art and Future Opportunities. Chapter 1: 3–18. ACS Symposium Series Vol. 1172. https://doi.org/10.1021/bk-2014-1172.ch001
Mogrovejo DC, Perini L, Gostin?ar C, Sep?i? K, Turk M, Ambroži?-Avguštin J, Brill FHH, & Gunde-Cimerman N. 2020. Prevalence of antimicrobial resistance and hemolytic phenotypes in culturable arctic bacteria. Front. Microbiol. 11: 570. https://doi.org/10.3389/fmicb.2020.00570
Naomi C, Suardana IW, & Suarsana IN. 2019. Isolated hemolysis profile of Streptococcus sp. isolation result from swine’s tonsil in slaughter house at Punggul and Bongkasa Village. JVAS. 2(2): 46–51. https://doi.org/10.24843/JVAS.2019.v02.i02.p01
Oktafiyanto MF, Munif A, & Mutaqin KH. 2018. Aktivitas antagonis bakteri endofit asal mangrove terhadap Ralstonia solanacearum dan Meloidogyne spp. [Antagonistic activities of mangrove endophytic bacteria against Ralstonia solanacearum and Meloidogyne spp.]. J. Fitopatol. Indones. 14(1): 23–29.
Thakkar P, Modi HA, & Prajapati JB. 2015. Isolation, characterization and safety assessment of lactic acid bacterial isolates from fermented food products. Int. J. Curr. Microbiol. App. Sci. 4(4): 713–725.
Triwidodo H & Listihani. 2021. Isolation, selection and determination of endophytic bacteria from bamboo, gamal, tulsi, and alamanda. Sustainable Environment Agricultural Science. 5(2): 151–162. https://doi.org/10.22225/seas.5.2.4068.151-162
Wang N, Liu M, Guo L, Yang X, & Qiu D. 2016. A novel protein elicitor (PeBA1) from Bacillus amyloliquefaciens NC6 induces systemic resistance in tobacco. Int. J. Biol. Sci. 12(6): 757–767. https://doi.org/10.7150/ijbs.14333
Wick R. 2010. Tobacco hypersensitivity; the first test to screen bacteria for pathogenicity. National Plant Diagnostic Network. 5(7): 3–4. https://www.npdn.org/sites/npdnd9b.ceris.purdue.edu/files/newsletter/NPDN%20News_July%202010.pdf